Published :
Updated :

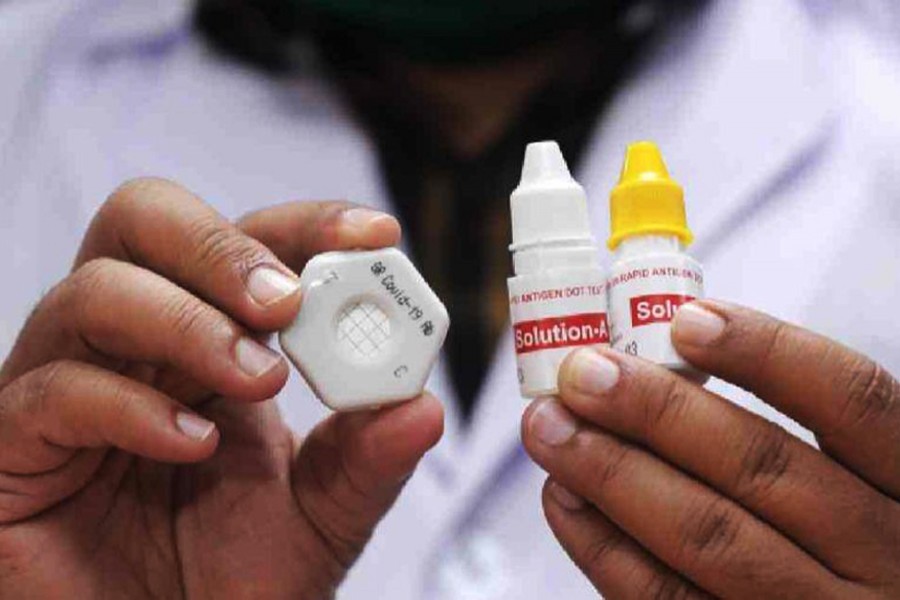
The Directorate General of Drug Administration has denied the Gonoshasthaya Kendra the permission to market coronavirus testing kits on the grounds that these have failed sensitivity test.
Minimum 90 per cent sensitivity is required for such kits while the ones of the Gonoshashthaya Pharmaceutical showed 69.7 per cent sensitivity during trial, the DGDA said in a letter to the organisation on Thursday.
The directorate also cited recommendation by a technical committee for the decision not to give Gonoshasthaya the permission to register its kits, reports bdnews24.com.
The Gonoshasthaya Kendra, however, termed the development “unfortunate”.
Muhibullah Khandaker, the coordinator of Gonoshasthaya’s Covid-19 Dot Blot Project, alleged in a statement that the directorate has dismissed the technical committee’s recommendation.
Bangabandhu Sheikh Mujib Medical University, which conducted the trial, had earlier said the kits were ineffective as they could accurately detect only 11-40 per cent of Covid-19 cases in the first two weeks after those patients showed symptoms.
The performance committee assessed 509 test kits before submitting the report.
The kits can identify antibodies but cannot differentiate immunoglobulin M, which is developed in the patient’s body at the beginning of infection from immunoglobulin G, which is found in the later stage of the infection, said BSMMU Vice-Chancellor Kanak Kanti Barua.
Gonoshasthaya submitted 200 homegrown kits to the BSMMU for trial on May 13. The conclusion made by the university after 34 days of assessment partly dimmed hopes for rapid testing kits developed by Gonoshasthaya.
Bangladesh relies only on the RT-PCR testing system for coronavirus diagnosis. It is viewed as the most reliable testing system in the world to detect the novel coronavirus called SARS-CoV-2, which causes the respiratory illness Covid-19.
The Gonoshasthaya announced the invention of the cheap and fast kits after the novel coronavirus pandemic hit the Bangladesh shores. The Directorate General of Drug Administration approved the import of reagents for the kits on Mar 19.
Gonoshasthaya Kendra Trustee Zafrullah Chowdhury, however, accused the DGDA of dallying in approving its kit. He alleged the directorate also declined to receive samples of its kit.
DGDA chief Maj Gen Mahbubur Rahman had urged Gonoshasthaya Kendra not to indulge in “spreading false news”.
After a long conflict, Gonoshasthaya received permission to submit samples of the rapid dot blot kits to the BSMMU for the assessment.


 For all latest news, follow The Financial Express Google News channel.
For all latest news, follow The Financial Express Google News channel.