Published :
Updated :

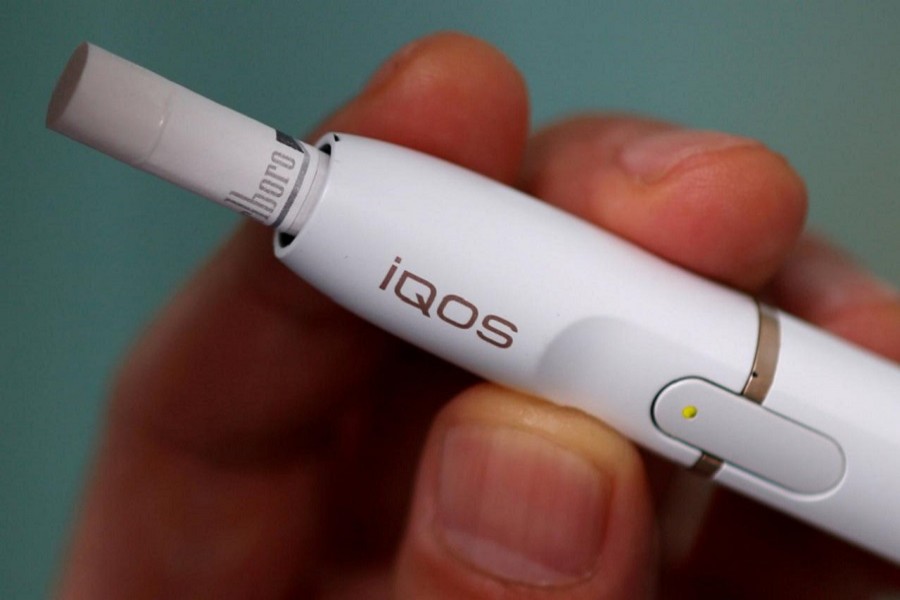
In a decision expected to test the Trump administration’s approach to tobacco regulation, US health advisers will vote this week on whether to allow Philip Morris International Inc (PM.N) to sell its novel iQOS tobacco device and claim it is less harmful than cigarettes.
The sleekly packaged little tube would not look out of place in an Apple store. It is designed to heat tobacco but not burn it. Most of the harmful chemicals in tobacco are released when tobacco is burned.
The advisers to the Food and Drug Administration will discuss the product on Wednesday and Thursday and recommend whether it should be cleared. The FDA is not obliged to follow the recommendations of its outside advisers but typically does.
If cleared, iQOS would become the first product to carry a modified-risk claim and could help advance the FDA’s proposed new approach to reducing the dangers of smoking.
For decades, US health agencies have worked to help Americans quit cigarettes to avoid the risks of lung cancer and other disease. National smoking rates have declined to near historic lows of around 15 per cent.
But in July, newly appointed FDA Commissioner Scott Gottlieb proposed reducing nicotine levels in cigarettes to “non-addictive” levels while increasing development of lower-risk alternatives. The policy assumes that some percentage of the population will be unable or unwilling to give up nicotine.
To make the new strategy succeed, the agency needs a stable of vetted, reduced-risk alternatives to cigarettes on the market. Philip Morris is one of the few companies that can finance such a long and expensive development process. It has spent close to $3 billion on reduced-risk products.
“If this application fails, it will be clear that this is an expensive, wasteful, regulatory dead end,” said Clive Bates, a tobacco expert who runs the consulting firm Counterfactual and advocates for alternative nicotine products, reports Reuters.
Bates and others argue that if Philip Morris cannot win FDA clearance for a modified-risk product, no one will be able to.
Other companies have submitted modified-risk products for FDA review. 22nd Century Group Inc (XXII.A), which genetically engineers tobacco plants to have lower nicotine levels, seeks clearance for its “Brand A” very low nicotine cigarettes. R.J. Reynolds Tobacco Co, owned by British American Tobacco Plc (BATS.L), is seeking clearance for six snus products - a moist smokeless tobacco pouch placed under the lip - under the Camel brand.


 For all latest news, follow The Financial Express Google News channel.
For all latest news, follow The Financial Express Google News channel.